An examination on the selection of a logistics service provider for the Life Sciences and Healthcare (LSHC) supply chain, illustrating flexibility, quality assurance and compliance and how cost effectiveness can help margins
by Fabio Mioli, DHL Supply Chain EMEA
 The line between a company’s internal operations and its external environment are becoming increasingly blurred. No area exemplifies this better than the Life Sciences and Healthcare (LSHC) supply chain, where manufacturers have to coordinate their own activities with those of partner organizations, healthcare providers, customers and patients. Without a clear understanding of the context surrounding the process of delivering a drug to market (i.e. changes in the distribution models and channels, mergers and acquisitions, increased market penetration of generic medicines) and changes to the market itself, the chain can soon become a tangled web.
The line between a company’s internal operations and its external environment are becoming increasingly blurred. No area exemplifies this better than the Life Sciences and Healthcare (LSHC) supply chain, where manufacturers have to coordinate their own activities with those of partner organizations, healthcare providers, customers and patients. Without a clear understanding of the context surrounding the process of delivering a drug to market (i.e. changes in the distribution models and channels, mergers and acquisitions, increased market penetration of generic medicines) and changes to the market itself, the chain can soon become a tangled web.
Outsourcing
Many companies in sectors such as fast moving consumer goods (FMCG), electronics, and automotive focus on core activities, while non-core logistics activities are often outsourced to logistics service providers (LSPs). It is estimated that about 50% of global logistics are now outsourced in many sectors. However, in several industries, including the Life Sciences and Healthcare (LSHC) industry, it is generally a smaller proportion of non-core logistics requirements that are outsourced. In relation to other industries, the outsourcing of logistics activities in pharmaceuticals is limited.
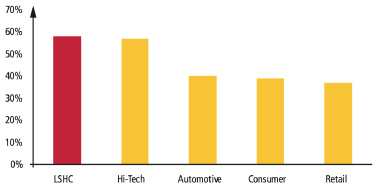 The graph shows that spend for outsourcing logistics is smaller and more fragmented within LSHC compared to other industries, suggesting that outsourcing acceptance within LSHC is lower. But the outsourcing wind of change is blowing. Five years ago, there were few examples of large LSHC companies outsourcing distribution operations to LSP partners. Manufacturers typically built large, dedicated warehouse facilities to store their products prior to shipping to their customers. But by August 2011, all top 10 global pharmaceutical manufacturers had outsourced at least part of their distribution operations across the globe, and there are more in the process of evaluating potential partners. Furthermore, companies entering emerging markets are increasingly looking to LSPs to support supply chain operations from the outset. Why the sudden change? Manufacturers are making constant strategic evaluations of their risks and challenges, and coming to a common conclusion on how best to improve their supply chains in response to various drivers, including increasing levels of global competition, lower margins, complex regulatory requirements, the costs of bringing drugs to market, generic competition, product and supply chain complexity. In addition, as a result of strong competition within the warehousing and transportation sector, LSPs are continuously looking for new markets. They consider the LSHC industry a commercially interesting playing field and are investing in capabilities to exploit these opportunities.
The graph shows that spend for outsourcing logistics is smaller and more fragmented within LSHC compared to other industries, suggesting that outsourcing acceptance within LSHC is lower. But the outsourcing wind of change is blowing. Five years ago, there were few examples of large LSHC companies outsourcing distribution operations to LSP partners. Manufacturers typically built large, dedicated warehouse facilities to store their products prior to shipping to their customers. But by August 2011, all top 10 global pharmaceutical manufacturers had outsourced at least part of their distribution operations across the globe, and there are more in the process of evaluating potential partners. Furthermore, companies entering emerging markets are increasingly looking to LSPs to support supply chain operations from the outset. Why the sudden change? Manufacturers are making constant strategic evaluations of their risks and challenges, and coming to a common conclusion on how best to improve their supply chains in response to various drivers, including increasing levels of global competition, lower margins, complex regulatory requirements, the costs of bringing drugs to market, generic competition, product and supply chain complexity. In addition, as a result of strong competition within the warehousing and transportation sector, LSPs are continuously looking for new markets. They consider the LSHC industry a commercially interesting playing field and are investing in capabilities to exploit these opportunities.
Pressure on margins
 Like many industries, global competition is driving market consolidation in healthcare. In 1998, the revenue of the top 10 pharmaceutical manufacturers was 25% of the global market. In 2010, it had risen to 58%. The continued pace of mergers and acquisitions has dramatically increased the awareness of the need for flexibility. Companies see the benefit of shifting responsibility to a global LSP who is likely to have access to more facilities and resources, and consequently be able to quickly respond to changing business needs. As an alternative to investing in upgrades, more manufacturers see the benefits in leveraging the existing asset and risk management infrastructure of an LSP. As global competition increases, LSHC manufacturers are painfully aware of the pressures on profit margins. At the same time, traditional strategies to boost revenue and profits are proving less effective: public and private purchasers are increasingly resistant to price increases.
Like many industries, global competition is driving market consolidation in healthcare. In 1998, the revenue of the top 10 pharmaceutical manufacturers was 25% of the global market. In 2010, it had risen to 58%. The continued pace of mergers and acquisitions has dramatically increased the awareness of the need for flexibility. Companies see the benefit of shifting responsibility to a global LSP who is likely to have access to more facilities and resources, and consequently be able to quickly respond to changing business needs. As an alternative to investing in upgrades, more manufacturers see the benefits in leveraging the existing asset and risk management infrastructure of an LSP. As global competition increases, LSHC manufacturers are painfully aware of the pressures on profit margins. At the same time, traditional strategies to boost revenue and profits are proving less effective: public and private purchasers are increasingly resistant to price increases.
New blockbuster drugs, which typically generate more than $1 billion of revenue for their owner each year, are fewer and further apart. Margin pressures are driving manufacturers to seek cost reductions in commercial operations. When healthcare companies examine their operations, often they report finding inefficiencies and high cost structures, driven by under-utilized facilities, high labor costs and expensive fixed assets. At the same time, supply chain budgets for high cost products are a small percentage of the value of the inventories. This explains why finding a strategic partner to navigate the complexity, and to provide flexibility, risk management, and best practice is an even more important consideration than cost.
Flexibility
 LSHC companies need flexibility, and the smartest way to deliver it is to partner with a LSP who already has it. The increasing complexity of supply chains, the real and potential changes in products and customers, and the myriad of local regulatory requirements in each geography, require industry and local expertise. It is typically quicker, easier and less risky to partner with a provider that can create a customized solution to address their company’s unique business needs, rather than building expertise in-house. Fluctuation in demand for branded and generic products, and changes in distribution channels are driving the continued evolution of supply chain models.
LSHC companies need flexibility, and the smartest way to deliver it is to partner with a LSP who already has it. The increasing complexity of supply chains, the real and potential changes in products and customers, and the myriad of local regulatory requirements in each geography, require industry and local expertise. It is typically quicker, easier and less risky to partner with a provider that can create a customized solution to address their company’s unique business needs, rather than building expertise in-house. Fluctuation in demand for branded and generic products, and changes in distribution channels are driving the continued evolution of supply chain models.
For example, the loss of patent protection is impacting the supply chains of manufacturers. 70% of generic drugs are now delivered direct to retailers in the United States; in Europe the Direct-to-Pharmacy (DTP) model has become mandatory for generics manufacturers and has been increased for brand-name drugs by a double digits ratio. Meanwhile, new direct-to-patient, high-cost specialist therapies are also causing manufacturers to reconsider how they take products to market in order to better respond to consumer demand. Differences in supply chain configurations between different products and the likelihood of new supply chain models have caused savvy, forward-looking manufacturers to seek out greater flexibility in their supply chains.
While margin pressures demand efficiency improvements, at the same time, supply chains must deliver increased flexibility to address mounting complexity and risk, and ensure regulatory compliance. A smart way to meet these divergent objectives is by leveraging the state-of-the-art technology, people and expertise of an LSP to support manufacturers.
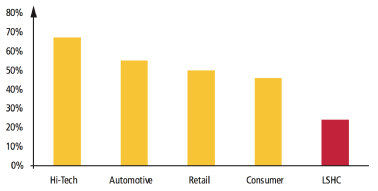
Almost all the top global pharmaceutical manufacturers have begun outsourcing all, or part, of their warehousing and transportation operations. Looking at the top 20 global pharmaceutical manufacturers, the estimation of percentage of sites outsourced to LSPs is more than 60%, while mature markets such as North America and Europe have a higher percentage of in-house solutions. The degree of cost sharing that is desirable varies by manufacturer. For example, outsourced warehousing and transportation can be shared or dedicated. In a dedicated model, manufacturers can own or lease the facility, and outsource only the labor, processes and technology. Or, they can outsource it all for a more complete solution and fewer assets on the balance sheet.
Depending on the financial details of existing leases or ownership, and the manufacturer’s forecasts, it can make sense to sell existing dedicated facilities and leverage an LSP’s shared-use supply chain solution. In this case, an outsourced facility or transportation mode is shared between multiple manufacturers. Benefits include lower costs, flexibility and scalability.
Product characteristics, regulatory requirements, cost considerations and the manufacturer’s prioritization of flexibility and cost determine which warehousing and transportation solution is the best fit for each product in a manufacturer’s portfolio.
Quality assurance and compliance
 Non-core logistics activities in many cases are still operated by the LSHC companies themselves, to a much greater degree than in other industries. This is because the healthcare industry is a much more conventional industry than some, with logistics activities and regulations perceived as being very complex. Transportation, storage, handling and production activities are strictly regulated by national governments. So it’s not hard to see why pharmaceutical companies have traditionally wanted direct control over their end-to-end supply chain. However, LSPs are experts in regulatory and compliance knowledge. National, state and local regulatory bodies create a bewildering range of requirements that a manufacturer’s small supply chain team can struggle to stay up-to-date with, let alone learn when entering a new geographic market. LSPs with experience in that market can leverage knowledge across their customer bases.
Non-core logistics activities in many cases are still operated by the LSHC companies themselves, to a much greater degree than in other industries. This is because the healthcare industry is a much more conventional industry than some, with logistics activities and regulations perceived as being very complex. Transportation, storage, handling and production activities are strictly regulated by national governments. So it’s not hard to see why pharmaceutical companies have traditionally wanted direct control over their end-to-end supply chain. However, LSPs are experts in regulatory and compliance knowledge. National, state and local regulatory bodies create a bewildering range of requirements that a manufacturer’s small supply chain team can struggle to stay up-to-date with, let alone learn when entering a new geographic market. LSPs with experience in that market can leverage knowledge across their customer bases.
As global competition increases, the need to focus scarce resources on core capabilities and leverage the expertise of others intensifies. The perspectives gained from a few people working for one manufacturer are not as advantageous as drawing on the collective knowledge of thousands working for many.
The potential to leverage expertise goes beyond people and knowledge. Healthcare companies indicated a wide variety of gaps in their existing operations processes and infrastructure, including inadequate controls, a lack of cold chain or controlled substance capacity, and outdated facilities or technology platforms.
LSHC sub-sectors
In analyzing the logistics priorities and requirements of LSHC companies it is helpful to divide them into four main sub-sectors:
Patented drugs
An R&D intensive sub-industry, dominated by multi-national companies, which covers about 50% of the European market. New product launches are extremely important, and the time to market determines the ultimate competitive advantage a company can create.
Non-patented drugs
Also known as generics and over-the-counter (OTC). Dominated by companies that are specialists in the sales of non-intensive drugs and focus on local markets. These products have significantly lower profit margins than patented products. Competition is strong and supply chain efficiency determines competitive advantage.
Biopharmaceuticals
Expected to grow and deliver significant advances in healthcare. Products include vaccines, hormones and enzymes which often require special transportation and storage conditions that have to be maintained from the production release up to the final distribution).
Medical devices
 Often sold through B2B channels. Growth is expected. This sub-sector covers a wide range of medium and high-technology products such as surgical and medical instruments, electronic medical equipment, X-ray, CAT and MRI equipment, dental equipment, diagnostic products and medical disposables. Customer service is crucial, as is production flexibility. Products are characterized by their high value, density and stock policy, an important factor to prevent extreme high working capital in inventory.
Often sold through B2B channels. Growth is expected. This sub-sector covers a wide range of medium and high-technology products such as surgical and medical instruments, electronic medical equipment, X-ray, CAT and MRI equipment, dental equipment, diagnostic products and medical disposables. Customer service is crucial, as is production flexibility. Products are characterized by their high value, density and stock policy, an important factor to prevent extreme high working capital in inventory.
Key points
As an LSHC company when you outsource the day-to-day logistics operation, leaving a certain dependency with the LSPs it is vital to:
- sustain specific supply chain expertise within your company
- have a clear and dedicated management structure towards the LSP on both strategic and tactical levels
- ensure a high quality of (electronic) information exchange, which is essential for a successful partnership.
To guarantee a high-quality operation, the focus should be on creating a partnership with the LSP rather than a ‘commodity’ cost reduction. A valuable tool for creating this mutual understanding and partnership is the Service Level Agreement (SLA). An SLA consists of a main (legal) document and several schedules concerning performance levels, logistics services, transition management, relationship management etc. It is not just a legal document, but should serve as a directive for day-to-day business processes as well.
Be aware, warehouse outsourcing does not automatically bring savings. Efficiency savings have to be actively looked for. This relates to opportunities such as “shared warehousing”, leveraging seasonality, and workload management with other businesses. Many companies do not assign all their logistics costs one-to-one on a supply chain budget. Outsourcing provides full transparency of total supply chain costs, which could be higher than the current perception.
Do not outsource to solve a disorder in operational logistics. An LSP will not solve that (as the root of the problem is often of a non-supply chain nature) and will charge you for this additional cost, as a result of the disruption to the warehouse and distribution processes. In addition, outsourcing has a long-term component; it is a strategic decision that cannot be changed every two years.
The pricing model in the outsourcing contract deserves specific attention, as it directly influences the day-to-day relationship between the shipper and the LSP. It should support an optimal fit between the specific operational characteristics and the ability to manage and control the LSP, while maximizing the uniformity of the contract components.
Therefore the choice for the right cost drivers is crucial. It must:
- be a motivation to decrease overall costs
- be able to manage performance
- positively influence the ordering profile.
